April 14, 2022
Blockchain solutions for the cold chain
Life Science
Who deals with logistics and cold chain in the pharmaceutical market knows how many problems there are in managing the TOR or “Time Out of Range” that is, the maximum total time for which a given pharmacological product may be outside the set temperature range for its proper storage: a value calculated by subtracting from the original measure the times when the product remains outside a refrigerated area.
For the different operators of the supply chain and distribution of the drug in fact the main problem within the entire process is represented by the continuous control of the TOR, which must necessarily be measured and communicated during each individual activity of the cold chain. The cold chain is an indispensable process to maintain the characteristics of the drug during transport and thus ensure its pharmacological activity.
Cold chain of the drug & distributive logistics: the criticalities
The current logistic iter of measurement of the cold chain turns out difficult since most of the controls of the TOR are carried out manually and autonomously from every actor of the row, while maintaining thermosensitive pharmaceuticals at a constant temperature throughout the entire supply chain may not be guaranteed.
In the pharmaceutical sector, in fact, GCCMP, Good Cold Chain Management Practices, are in force, obliging each of the operators in the sector, during transport and stocking, to verify the integrity and stability of the product, as well as to the storage of medicinal products at the indicated temperatures, usually varying between 2 and 8,thus.
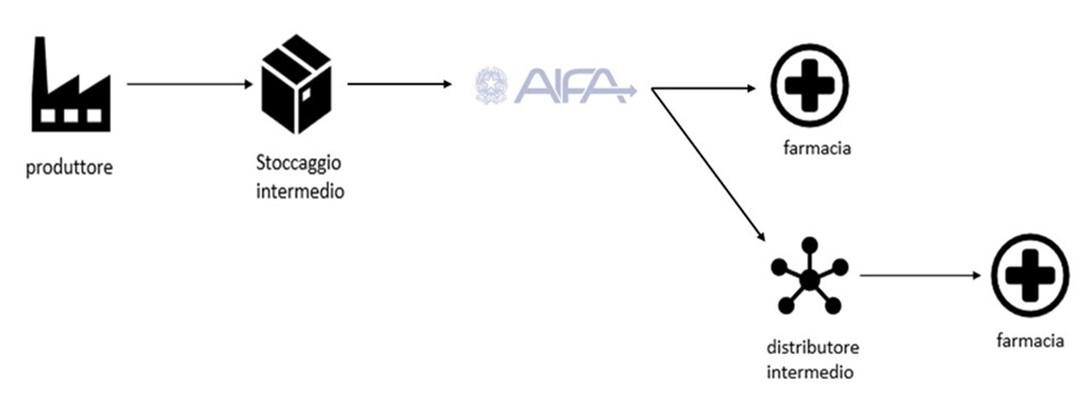
Among the three macro-phases of the logistics of pharmaceutical products, namely production, packaging and distribution, the latter has as main actors distributors and is affected by the necessary granting of the Marketing Authorisation AIC. After obtaining the AIC, granted by AIFA, Italian Agency of Medicine, the primary distributor sends the order to the wholesaler, secondary distributor, which has as its task to supply the final customer and then the market.
It is in this scenario that in response to the growing needs of traceability of the market, from the manufacturer to the final consumer, with the prospect of a complete and reliable reconstruction of the condition of the pharmaceutical product, was born the project of Cold Chain Platform.
The benefits of the new Cold Chain Platform for the total drug quality system
The latest temperature mapping services project was born from the common will of some companies, involved in the temperature-controlled supply chain of the pharmaceutical sector, to develop an operational design to guarantee the validity and objectivity of the data.
Among the main objectives is to reduce the cost items related to the monitoring of the temperature of the drug within each area of responsibility, together with the costs by the manufacturer for the management and control of the drug along the supply chain.
The Cold Chain Platform (CCP) developed on blockchain technology by JSB Solutions in collaboration with other companies belonging to the cold chain Harmaceutical and with the advice of the AFI, enters the field to improve the traceability of the pharmaceutical product along the whole chain, through the use of devices in network with a computer platform for data management; a distributed database and unique for all actors, developed to obtain:
Blockchain enables safe and unchangeable recording of data collected by devices for timely quality monitoring, which is measured, controlled, documented and validated. It is crucial that a cold chain is based on a quality management system.
The benefits for companies investing in new technology are undeniable in terms of:
The advantages of adopting the JSB blockchain platform for cold chain in pharma and beyond – just think of other perishable products such as food (fresh and frozen), photographic films or chemicals – not only involve the companies that implement it, but also affect the end user and are related to the reduction of risks related to counterfeiting and incorrect storage.
In addition, the patient who purchases the drug through the platform will be able to personally reconstruct and verify the history and condition of their drug.
Scopri di più
Vai al case study